Medical Device Failure
Due to the increased occurrence of defective implanted medical devices with lawsuits in the billions. There needs to be a process in which the manufacturer is held accountable to FDA safety regulations and provides safety assurance to the end-user of the implanted medical device. Currently, there is zero communication between user and the manufacturer or a governing body for recalls. Working with FDA Regulations this product assures compliance of the manufacturer. Provides the user with an assurance of accurate updates and recall warnings.
Scenario
You just had a medical device implanted in your body and you want to register it and track any concerns or recalls. Additionally, if you have a medical device that monitors or collects data it manages your personal information. A manufacturer can collect safety data.
MedDevTrack Case Study
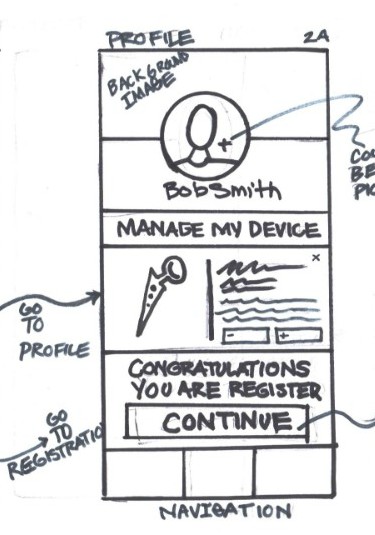
Sign Up Interaction
Short interaction video of 2 screen sign up process with overlays.
- Sign In Screen
- Sign Up Screen
- Profile Screen
- Health Info Screen
- Alert Screen
Scenario
- Medical device implanted, register it and keep personal info up-to-date
- Recieve related health info and current trusted news
- Manage Alerts for potential recalls
Needs:
- Compliance of the manufacturer to New FDA Regulations
- Communication channel between user and the manufacturer
- Provides the user with an assurance of accurate updates and recall warnings.
Pain points:
- No where to report issues
- Worry of giving away health data
- No communication with the manufacturer
Concerns:
- Accurate warnings or possible recall
- Security of patient data collected
- No accountability from manufacturer
Goals:
- To empower and engage the user to access and manage an implanted medical device data
- Keep their contact information current to receive updates or recall warnings
- Keep information private

Research: The auto industry for recalls and the oil industry for safety. Researched how users sign up for warranty. What users see as a credible and accurate information.
Insights: The lawsuits and millions of people suffering from defective medical devices there is a need for accountability and reform. New FDA Regulations will require it.
Principles:
- Simple and easy sign-up
- Ease of use to self-update contact and useful information
- Notifications accurate and subdued - Alerts that demand attention
Platforms:
- Cloud-based
- Desktop browser and Mobile App
- Connected Medical Device
My Role:
- Research
- UI Design
- User Experience methodology
Favorite Personas




Prototype/User Testing:
- Learned muted colors work better
- Menu clickable area larger
- Icons on buttons ease language barrier
Conclusions: The redesign went smoothly. The content management piece proved the most difficult with customizing the approval process involving legal and medical changes and maybe a sign off. The user testing went well users were generally happy. The individual health sites went well with the normal hiccups. Overall it was a positive experience had by all.
Results:
- 2.0 Site launched on-time, in 1 year with updates planned every 3 months
- Business units now have a publishing tool to the Internet
- Ask Jeeves search much more relevant and useful
Story Boarding-Wire Frames:
- Learned that less is more
- Concept different features
- Shaved off 3 clicks to sign up
Design Critique:
- Sign-In screen needed differentiation
- Smaller logo
- Use slide out for more info



